Molnupiravir / Yxosnpfci0qoym
As of June 25 2021 SARS-CoV-2 has infected over. Molnupiravir is currently also being assessed in newly hospitalised patients with COVID with this study aiming to find out if early molnupiravir treatment can reduce the time it.
Molnupiravir MK-4482EIDD-2801 is an investigational orally administered form of a potent ribonucleoside analog that inhibits the replication of SARS-CoV-2 the causative agent of COVID-19.

Molnupiravir. Molnupiravir was generally well tolerated with similar numbers of adverse events across all groups. In March 2021 the companies reported preliminary results from a Phase IIa trial of molnupiravir for Covid-19. Molnupiravir demonstrated activity in preclinical models of SARS-CoV-2 SARS-CoV-1 and MERS including prophylaxis cure and transmission prevention.
Based on our data we developed a model that describes effects on both efficiency and fidelity of RNA synthesis Fig. Molnupiravir een experimenteel antiviraal geneesmiddel is effectief tegen een aantal virussen waaronder coronavirussen en specifiek SARS-CoV-2. Molnupiravir a wide-spectrum antiviral that is currently in phase 23 clinical trials for the treatment of COVID-19 is proposed to inhibit viral replication by a.
A novel coronavirus originally identified in Wuhan City China was reported to the World Health Organization on 31 December 2019 and the associated disease has subsequently become a worldwide pandemic. Molnupiravir has been shown to be active in several preclinical models of SARS-CoV-2 including for prophylaxis treatment and prevention of transmission. Molnupiravir originally created by researchers at Emory University in Atlanta is given as four pills taken twice a day for five days.
Molnupiravir if approved would be the first orally active direct-acting antiviral drug for COVID a significant advance in fighting the pandemic. An experimental COVID-19 treatment pill called molnupiravir being developed by Merck Co Inc and Ridgeback Biotherapeutics LP is seen in this undated handout photo released by Merck Co Inc and. He said his team is now looking to conduct clinical trials for molnupiravir.
Populair-wetenschappelijk nieuws trivia braintainmentStudies lijken aan te tonen dat het coronamedicijn Molnupiravir werkt. The program has advanced into Phase 3 development as a therapeutic for COVID-19. Molnupiravir an Oral Antiviral Treatment for COVID-19.
We report the results of a Phase 2a trial evaluating the safety tolerability and antiviral efficacy of molnupiravir in the treatment of. Ridgeback has completed Phase 1 and Phase 2 studies. Molnupiravir has Phase IIa data showing it can reduce a patients viral load.
Background Easily distributed oral antivirals are urgently needed to treat coronavirus disease-2019 COVID-19 prevent progression to severe illness and block transmission of severe acute respiratory syndrome coronavirus 2 SARS-CoV-2. By eddyjoemd In COVID-19. Molnupiravir is a strong backbone drug candidate from which multiple combinations can be derived he added.
Molnupiravir has an attractive oral formulation ideal for outpatient use but a lack of long-term data may limit initial rollout to high-risk people. Binnen 5 dagen was het coronavirus verdwenen bij alle deelnemende patienten die Molnupiravir hadden gehad. Timelines as to when to use molnupiravir and the patients vaccination status are confounding factors in gathering efficacy evidence in the trial and in practice if it garners regulatory support.
From Press Release to Practice. Molnupiravir an oral ribonucleoside analogue is also being studied in the Phase. Molnupiravir is a promising and clever drug but we need more information.
Because molnupiravir has shown activity against strains of coronavirus that cause the common cold it may be studied for this purpose in the future or other viruses entirely. Given were still averaging 122 deaths a day from COVID in the UK despite high levels of vaccination a drug. Molnupiravir is therefore classified as a mutagenic nucleotide analogue.
Molnupiravir is a direct-acting oral broad-spectrum antiviral agent in clinical development as a treatment for COVID-19. On October 2 2021. An effective antiviral therapeutic has since been intensively sought.
Yesterday 100121 for historical context there was quite a stir after a press release by Merck Pharmaceuticals after reports that their new antiviral medication molnupiravir showed benefits in early COVID-19. Of the participants who received molnupiravir. Molnupiravir is the first oral direct-acting antiviral shown to be highly effective at reducing nasopharyngeal SARS-CoV-2 infectious virus and viral RNA and has a favorable safety and tolerability profile.
Virus isolation was 19 in the 800mg molnupiravir group compared to 167 in the placebo group at day three representing a statistically significant difference according to a Phase IIa preprint manuscript. Here we studied the underlying biochemical mechanisms with the purified RdRp complex of SARS-CoV-2. In a trial of 775 patients with mild-to-moderate COVID-19 who were considered higher risk for severe disease molnupiravir reduced hospitalization by 50.
Molnupiravir an oral antiviral treatment for COVID-19.

Molnupiravir Last Of The Small Molecule Coronavirus Hopes Science Aaas

Covid Molnupiravir Drugmaker Merck Seeks Marketing Approval Sortiraparis Com
A Pill To Treat Covid 19 We Re Talking About A Return To Maybe Normal Life Cnn
Lung Center Qmmc Call For Participants In Molnupiravir Trials Vs Covid 19
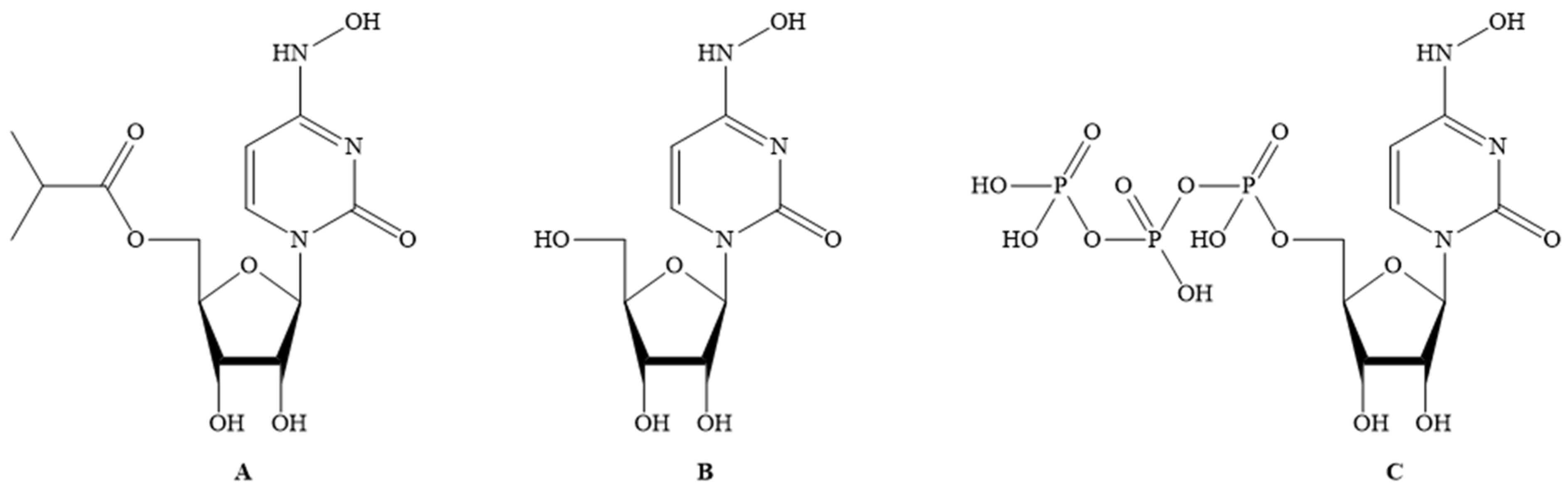
Molecules Free Full Text Discovery Development And Patent Trends On Molnupiravir A Prospective Oral Treatment For Covid 19
Covid 19 Uk Shows Interest In Antiviral Pill Molnupiravir After Trial Shows It Could Halve Hospitalisations And Deaths Uk News Sky News

From Merck S Molnupiravir To Glenmark S Nasal Spray Over 20 New Drugs In Pipeline For Covid 19